Abstract
Introduction:
CD19 CAR T-cell therapy has revolutionized outcomes for patients (pts) with relapsed, refractory (R/R) B-cell non-Hodgkin lymphoma (NHL). However, the vein-to-vein time for commercially approved CAR-T cell products and downregulation of the target receptor with CD19 CAR-T remain limitations of therapy. To improve clinical outcomes we utilized a lentiviral, bispecific, tandem anti-CD20/anti-CD19 (LV20.19) CAR T-cell in pts with R/R B-cell NHL with point of care manufacturing (MF) to limit time to infusion. For MF, we used IL-7+IL-15 for cell expansion with two arms comparing a flexible 8/12 day MF process versus (vs) a fixed 12 day platform. We hypothesized shorter MF would produce a more naïve CAR T-cell population improving clinical responses for R/R B-cell NHL.
Methods:
We conducted a Phase 1/2, multi-cohort, single center, prospective trial (NCT04186520) evaluating LV20.19 CAR T-cells at a fixed dose of 2.5x10e6 cells/kg for pts with R/R diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL). Cells were manufactured via CliniMACS Prodigy device. Pts were sequentially enrolled on the two arms: fixed 12-day MF vs flexible 8/12 day MF where cells were harvested on day 8 if target dose was met. The goal was fresh infusion for all pts with lymphodepletion initiated during the MF process. After safety run-in, we opened two Phase 1b expansion cohorts. Descriptive statistics were utilized for baseline characteristics and Fischer's exact test for comparison of categorical variables. Survival analyses were calculated utilizing the Kaplan-Meier method and differences between groups were evaluated via log-rank test.
Results:
In total, there were 21 pts with DLBCL or FL treated with LV20.19 CAR-T cells with 11 pts treated on the flexible 8/12 MF arm and 10 pts treated on the fixed 12-day MF arm. MF success was 100% and 18 (86%) pts received fresh cells without cryopreservation. All 11 pts on the flexible 8/12 arm met goal target dose at Day 8 of MF. The median age of all pts was 66 years (41-78). Median prior lines of therapy were 3 (2-11). Nine (43%) pts progressed after prior autologous transplant and 2 pts progressed after anti-CD19 CAR T-cell therapy (Table 1).
By cohort, there was no difference in day 28 overall response rates: 82% with an 8-day process vs 70% with a 12-day process (p=0.64). Both prior CAR treated pts progressed at day 28. Day 28 complete response (CR) rates trended higher in the 8-day arm (64% vs 20%, p=0.08). There was no difference in median duration of response (DOR); 6 months in the 8-day arm vs not reached in the 12-day arm (p=0.64). The 1-year OS for all pts was 61%. There were 4 non-relapse mortality events in responding pts beyond day 28 (1=therapy related MDS, 1=covid, 1=line associated sepsis, 1=pneumonia). More pts in the 8-day arm had CRS, 100% (n=11) with 1 grade 3 vs 70% (n=7) in the 12-day arm, all grade 1-2 (p=0.08). Immune effector cell associated neurotoxicity syndrome (ICANS) occurred in 2 pts in the 8-day arm, both grade 1-2 and in 4 pts in the 12-day arm (2=grade 2 and 2=grade 3).
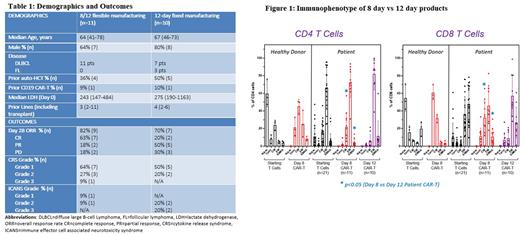
Immunophenotypically CAR T-cells harvested at day 8 had significantly higher numbers of central memory (CM) T-cells and lower terminally differentiated effector memory re-expressing CD45RA (EMRA) T-cells in both CD4 and CD8 compartments than CAR T-cells harvested at day 12 (Figure 1). Intra-patient immunophenotyping of 3 pts assigned to the 12-day MF arm demonstrated a similar shift with decreasing CM T-cells and increasing EMRA T-cells between day 7 (in-process testing) and day 12 (harvest).
Conclusions:
Bispecific LV20.19 CAR T-cells are safe and efficacious for pts with R/R B-cell NHL. Shorter MF produced a more naïve CAR T-cell product with no difference in toxicity profile. Higher percentage of CM T-cells and lower EMRA T-cells were observed earlier in the MF process both between the two cohorts and intra-patient. While CR rates were higher in the 8-day arm, duration of response was similar in both groups. These data confirm that a shorter MF process is feasible and improves immunophenotype of the final CAR product without impacting safety. Future studies will utilize an 8-day MF process.
Disclosures
Shah:Lilly Oncology: Consultancy, Honoraria; TG therapeutics: Consultancy; Kite Pharma: Consultancy; Novartis: Consultancy; Bristol Myers Squibb: Consultancy; Incyte Corporation: Consultancy, Honoraria, Speakers Bureau; Miltenyi Biotec: Consultancy, Research Funding; Epizyme: Consultancy. Hari:Sanofi: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Novartis: Honoraria; BMS: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Incyte: Honoraria; Kite: Consultancy, Honoraria; GlaxoSmithKline: Honoraria; AbbVie: Honoraria; Pharmacyclics: Consultancy; Millennium: Research Funding; Spectrum Pharmaceuticals: Research Funding; Iovance: Current Employment. Schneider:Lentigen Technology, a Miltenyi Biotec Company: Current Employment. Johnson:Miltenyi Biotec: Research Funding. Fenske:Adaptive Biotechnologies: Consultancy, Speakers Bureau; Beigene: Consultancy; Bristol-Meyers-Squibb: Consultancy, Speakers Bureau; CSL Therapeutics: Consultancy; Karyopharm: Consultancy; Kite (Gilead): Consultancy, Speakers Bureau; MorphoSys: Consultancy, Speakers Bureau; Pharmacyclics (AbbVie): Consultancy; SeaGen: Consultancy, Speakers Bureau; Servier Pharmaceuticals: Consultancy; TG Therapeutics: Consultancy, Speakers Bureau; ADC Therapeutics: Consultancy, Speakers Bureau; Astrazeneca: Speakers Bureau; Sanofi: Speakers Bureau. Hamadani:Incyte Corporation: Consultancy; Astellas Pharma: Research Funding; Kadmon: Consultancy; Sanofi Genzyme: Speakers Bureau; Abbvie: Consultancy; Medical University of Wisconsin: Current Employment; MorphoSys: Consultancy; Kite: Consultancy; Genmab: Consultancy; SeaGen: Consultancy; Gamida Cell: Consultancy; Novartis: Consultancy; Legend Biotech: Consultancy; ADC Therapeutics: Consultancy, Research Funding, Speakers Bureau; Omeros: Consultancy; Takeda: Research Funding; Spectrum Pharmaceuticals: Research Funding; AstraZeneca: Speakers Bureau; BioGene: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal